
Back ثنائي الغوانوسين أحادي الفوسفات الحلقي Arabic سیکلیک دی-جیامپی AZB سیکلیک دی-جیامپی Persian Di-guanosine monophosphate cyclique French Cyclisch diguanylaat Dutch Ciklični di-GMP Serbo-Croatian Cyklický di-GMP Slovak Ciklični di-GMP Serbian
| |
| Names | |
|---|---|
| Systematic IUPAC name
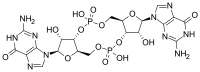
(2R,3R,3aS,7aR,9R,10R,10aS,14aR)-2,9-Bis(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,5,10,12-tetrahydroxyoctahydro-2H,5H,7H,12H-5λ5,12λ5-difuro[3,2-d:3′,2′-j][1,3,7,9,2,8]tetraoxadiphosphacyclododecine-5,12-dione | |
| Other names
Cyclic diguanylate; 3',5'-Cyclic diguanylic acid; c-di-GMP; 5GP-5GP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H24N10O14P2 | |
| Molar mass | 690.09 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyclic di-GMP (also called cyclic diguanylate and c-di-GMP) is a second messenger used in signal transduction in a wide variety of bacteria.[1] Cyclic di-GMP is not known to be used by archaea, and has only been observed in eukaryotes in Dictyostelium.[2] The biological role of cyclic di-GMP was first uncovered when it was identified as an allosteric activator of a cellulose synthase found in Gluconacetobacter xylinus in order to produce microbial cellulose.[3]
In structure, it is a cycle containing only two guanine bases linked by ribose and phosphate.
- ^ Tamayo R, Pratt JT, Camilli A (2007). "Roles of cyclic diguanylate in the regulation of bacterial pathogenesis". Annual Review of Microbiology. 61: 131–148. doi:10.1146/annurev.micro.61.080706.093426. PMC 2776827. PMID 17480182.
- ^ Chen ZH, Schaap P (August 2012). "The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium". Nature. 488 (7413): 680–683. Bibcode:2012Natur.488..680C. doi:10.1038/nature11313. PMC 3939355. PMID 22864416.
- ^ Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, et al. (1987). "Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid". Nature. 325 (6101): 279–281. Bibcode:1987Natur.325..279R. doi:10.1038/325279a0. PMID 18990795. S2CID 4305512.